Diagnosis of Tuberculosis in bovine animals
Infection with Mycobacterium tuberculosis complex (M. bovis, M. caprae, M. tuberculosis) is included in the Regulation (EU) 2016/429 on transmissible animal diseases (“Animal Health Law”) that was adopted by the European Parliament and the Council in March 2016 (replacing the Council Directive 63/432/CEE) and it has been applicable since 21st April 2021. In bovine animals, diagnostic methods for the granting and maintenance of disease-free status [Commission Delegated Regulation (EU) 2020/689] and movement of animals within the Union [Commission Delegated Regulation (EU) 2020/688], are the tuberculin skin tests and the gamma-interferon assay.
According to article 6 of the Delegated Regulation 2020/689 regarding diagnostic methods (“the cascade principle”), Standard Operation Procedures (SOP) for in vivo intradermal tuberculin tests protocol (SOP/001/EURL) and in vitro gamma-interferon detection assay (SOP/004/EURL and SOP/006/EURL) are available at the EU-RL website. Both in vivo and in vitro assays are performed using purified protein derivatives (PPDs) obtained from the heat-treated products of growth and lysis of M. bovis (bovine PPD) and M. avium (avian PPD).
Intradermal tuberculin tests
The intradermal tuberculin test has been used for ante-mortem diagnosis of latent and active TB in humans and animals for more than 100 years. The test is based on the capacity of the PPDs to reveal a delayed hypersensitivity in a previous infected animal when they are intradermally inoculated. Finland was the first country in the late 1890s to start a bovine TB eradication campaign using the tuberculin test [1]. Once bovine TB program based on a test and slaughter policy began, the incidence of clinical TB decreased since a high proportion of infected cattle was removed. Other countries gradually applied eradication programs using different methodologies of the tuberculin test (ophthalmic and palpebral test, Stormont test, vulval test, etc.) that were subsequently discarded. Neck was finally selected as the site of tuberculin injection in the Member States (MS) due to the higher sensitivity (Se) and specificity (Sp) values in cattle in comparison with the caudal fold [2].
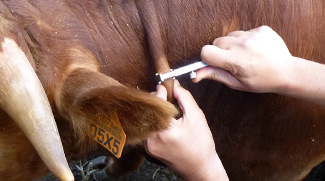
Intradermal tuberculin test measure. VISAVET-UCM.
The Single Intradermal Tuberculin (SIT) test measures the cell-mediated delayed type hypersensitivity against bovine PPD injected in the mid-cervical region (Member States) or in the caudal skin fold (United States, Canada and New Zealand) whereas the Single Comparative Intradermal Tuberculin (SCIT) test compares the response against bovine PPD and avian PPD in the cervical region with the aim of increasing the Sp. According to the protocol of the EU-RL (SOP/001/EURL), the injection site should be clipped and cleansed and injection site shall be situated at the border of the anterior and middle third of the neck since it has been demonstrated that maximize the test Se [16]. Afterwards skin fold thickness should be measured using a caliper. The tuberculin can be injected using different syringes as the McLintock (Bar Knight McLintock Limited, UK) and Dermojet (Akra Dermojet, France) syringes. A maximum volume of 0.2 ml containing a minimum of 2,000 IU of bovine and avian PPDs should be injected at cervical site. A small pea-like swelling in each site should be palpated by the veterinary practitioner to confirm the correct intradermal injection. When both avian and bovine PPDs are injected in the same animal, the site for injection of avian PPD shall be about 10 cm from the crest of the neck and the site for the injection of bovine PPD and about 12.5 cm lower on a line roughly parallel with the line of the shoulder or on different sides of the neck. In young animals in which there is not room to separate the sites on one side of the neck, one injection shall be made on each side of the neck at identical sites in the centre of the middle third of the neck. To ensure equivalent test Se at both avian and bovine sites, it has been suggested that both PPDs should be located on a line that is parallel to the angle of the shoulder [2][3]. After 72 hours (±4 hours), the skin fold thickness at each injection site should be remeasured by the same veterinary and interpretation should be performed according to the SOP.
Several factors related to the immunological response (early infection, anergy or concurrent immunosuppression), to the PPDs (expired product, product stored under inappropriate conditions, manufacturing errors, low potency) or to the methodology (doses, site of injection, inexperience) might cause false negative results [4][5]. On the other hand, co-infection or pre-exposure to other related non-tuberculous mycobacteria have been reported as a potential cause of a false positive result due to the similar antigenic composition of these bacteria [4].
Biological potency of the tuberculins is critical for the outcome of the intradermal test. In fact, a significant difference in the number of reactors detected using high and low potency tuberculins has been reported [3]. Production of PPD tuberculins are standardised and regulated by the EU. Manufacture must fulfil the Good Manufacturing Practice conditions and comply with the European Pharmacopeia (EP) and the WOAH Terrestrial Manual requirements [2][6]. The protein content of the tuberculins is not correlated with the biological activity and therefore, potency assays of the tuberculin batches must be performed in guinea pigs and cattle [7]. The requirement to check the potency in the bovine bio-assay is also performed according to the WOAH and EP recommendations [2]. Currently, only some laboratories are carrying out potency tests of bovine PPDs from different manufacturers in cattle.
Several studies have been carried out in the last decades to evaluate the performance of the intradermal tuberculin test in cattle under different epidemiological situations and using different antigens [8]. Reactivity against the tuberculin usually develops between 3 and 6 weeks post-infection. In fact, some studies have suggested the pre-allergic phase as a cause of false negative reactions and therefore, the lack of Se in recently infected animals [9][10].
Reported Se of the SIT test in cattle ranged between 80.2% and 100% although it was evaluated under specific epidemiological circumstances [5]. More recently and using a Bayesian approach, the estimate Se of the SIT test ranged between 53% (27.3-81.5, 95% CI) and 69.4% (40.1-92.2, 95% CI) depending on the interpretation criteria used [11]. The results from this study highlighted the low Se of the intradermal test after the disclosure test. Reported Sp of the SIT test ranged between 55.1% and more than 99% showing a median value over 95% [5][12][13].
Sensitivity of the SCIT test is lower than that achieved using the SIT test. Reported values ranged between 52% and 100% [5][13]. In the presence of interference factors (e.g. infection by environmental mycobacteria), comparative interpretation increases the Sp (median of 99.5%) [5][13].
Interferon-gamma assay

Interferon-gamma immunoassay. VISAVET-UCM.
The in vitro interferon-gamma (IFN-γ) assay developed in Australia in the late 1980s [14] is recommended by the WOAH since 1996 (WOAH Manual of Diagnostic Tests and Vaccines for Terrestrial Animals) as ancillary laboratory-based test to the tuberculin intradermal test. Most of the bovine TB control programmes rely on the use of the IFN-γ assay as parallel test to the intradermal test to maximise the detection of infected animals. The assay was accepted for use as ancillary test to the intradermal test in the European Union since 2002 [Council Directive 64/432/EEC, amended by (EC) 1226/2002] and currently it is included in the Commission Delegated Regulation (EU) 2020/689 for granting and maintaining disease-free status and in the Delegated Regulation (EU) 2020/688 for movement of animals. The SOP (SOP/004/EURL and SOP/006/EURL) are available at the EU-RL website.
The IFN-γ assay is performed in two stages. Firstly, blood samples are collected using heparinized tubes and transported to the laboratory where they are incubated at 37 ºC in humidified atmosphere after stimulation with specific antigens such as PPDs or antigen cocktails to stimulate the release of IFN-γ by sensitised T lymphocytes. Several parameters of the blood incubation have recently been optimised to increase the flexibility and ease of use of the assay [15]. At the second stage, samples are centrifuged and the resultant plasma is used for detection of the released IFN-γ by a commercial enzyme sandwich immunoassay. The interpretation criteria of the IFN-γ assay can be adapted based on the epidemiological situation, disease prevalence and the stage of the bovine TB control program. Besides the EU-approved use as a parallel test to the intradermal tuberculin test to maximise Se, many countries have adopted protocols for the use of the IFN-γ assay as a serial test to the skin test in order to increase the Sp. An overview of the different cut-off criteria for the IFN-γ assay in the European Union was published by the EFSA [17]. Field studies carried out in the last two decades with the aim of comparing the diagnostic performance of the tuberculin intradermal test and the IFN-γ assay, demonstrated the higher Se of the IFN-γ test. Nevertheless, the Sp achieved was similar or slightly lower than that of the SIT, and lower than the Sp of the SCIT test. A meta-analysis of 15 field studies conducted between 1991 and 2006, showed an estimated median Se of 87.6% with a range between 73% and 100% and a Sp of 96.6% with a range of 85% and 99.6% for the IFN-γ assay [5]. The higher Se of the IFN-γ test compared to the skin test is likely due to the fact that the IFN-γ test detects TB infected animals as early as 14 days following infection [9] and 60-120 days earlier than the SCIT test [10].
References
- Francis, J. 1958. Tuberculosis in animals and a man. London: Cassell.
- Good, M., Duignan, A. 2011. Perspectives on the History of Bovine TB and the Role of Tuberculin in Bovine TB Eradication. Veterinary Medicine International, 410470.
- Good, M., Clegg, T.A., Costello, E., More, S.J. 2011. The comparative performance of the single intradermal test and the single intradermal comparative tuberculin test in Irish cattle, using tuberculin PPD combinations of differing potencies. Vet. J., 190:e60-e65.
- Humblet, M.F., Walravens, K., Salandre, O., Boschiroli, M.L., Gilbert, M., Berkvens, D., Fauville-Dufaux, M., Godfroid, J., Dufey, J., Raskin, A., Vanholme, L., Saegerman, C. 2011. Monitoring of the intra-dermal tuberculosis skin test performed by Belgian field practitioners. Research in Veterinary Science, 91:199-207.
- Rua-Domenech, R., Goodchild, A.T., Vordermeier, H.M., Hewinson, R.G., Christiansen, K.H., Clifton-Hadley, R.S. 2006. Ante mortem diagnosis of tuberculosis in cattle: A review of the tuberculin tests, gamma-interferon assay and other ancillary diagnostic techniques. Res.Vet.Sci., 81:190-210.
- WOAH. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Mammalian tuberculosis (infection with Mycobacterium tuberculosis complex). Chapter 3.1.13.
- Haagsma, J. 1986. Potency testing of bovine tuberculins. Dev Biol Stand, 58(Pt B):689-694.
- Bezos, J., Casal, C., Romero, B., Schroeder, B., Hardegger, R., Raeber, A.J., Lopez, L., Rueda, P., Dominguez, L. 2014. Current ante-mortem techniques for diagnosis of bovine tuberculosis. Research in Veterinary Science. Suppl:S44-52.
- Buddle, B.M., de Lisle, G.W., Pfeffer, A., Aldwell, F.E., 1995. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine, 13:1123-1130.
- Lilenbaum, W., Schettini, J.C., Souza, G.N., Ribeiro, E.R., Moreira, E.C., Fonseca, L.S. 1999. Comparison between a gamma-IFN assay and intradermal tuberculin test for the diagnosis of bovine tuberculosis in field trials in Brazil. Zentralblatt fur Veterinarmedizin. Reihe B. Journal of veterinary medicine, Series B46:353-358.
- Alvarez, J., Bezos, J., de, J.L., Vordermeier, M., Rodriguez, S., Fernandez-De-Mera, I.G., Mateos, A., Dominguez, L. 2012. Diagnosis of Tuberculosis in Camelids: Old Problems, Current Solutions and Future Challenges. Transbound. Emerg. Dis. 59, 1-10.
- Alvarez, J., Perez, A., Bezos, J., Marques, S., Grau, A., Saez, J.L., Minguez, O., de Juan, L., Dominguez, L. 2012. Evaluation of the sensitivity and specificity of bovine tuberculosis diagnostic tests in naturally infected cattle herds using a Bayesian approach. Veterinary Microbiology, 155:38-43.
- Schiller, I., Oesch, B., Vordermeier, H.M., Palmer, M.V., Harris, B.N., Orloski, K.A., Buddle, B.M., Thacker, T.C., Lyashchenko, K.P., Waters, W.R. 2010. Bovine tuberculosis: a review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound. Emerg. Dis, 57: 205-220.
- Wood, P.R., Jones, S.L. 2001. BOVIGAM: an in vitro cellular diagnostic test for bovine tuberculosis. Tuberculosis. (Edinb.), 81:147-155.
- Schiller, I., Waters, W.R., Vordermeier, H.M., Nonnecke, B., Welsh, M., Keck, N., Whelan, A., Sigafoose, T., Stamm, C., Palmer, M., Thacker, T., Hardegger, R., Marg-Haufe, B., Raeber, A., Oesch, B. 2009. Optimization of a whole-blood gamma interferon assay for detection of Mycobacterium bovis-infected cattle. Clin. Vaccine Immunol, 16:1196-1202.
- Casal C., Alvarez J., Bezos J., Quick H., Diez-Guerrier A., Romero B., Saez JL., Liandris E., Navarro A., Perez A., Dominguez L. de Juan L. 2015. Effect of the inoculation site of bovine purified protein derivative (PPD) on the skin fold thickness increase in cattle from officially tuberculosis free and tuberculosis-infected herds. Preventive Veterinary Medicine, 121(1-2):86-92.
- EFSA. Scientific Opinion on the use of a gamma interferon test for the diagnosis of bovine tuberculosis. 2012. EFSA Journal, 10(12):2975.
