Biofilms and their impact on food industry
A biofilm is a complex community of microorganisms that are embedded in a matrix of extracellular polymeric substances they have produced, and they are attached to either an inert or living surface and formed by one or more microbial species (Carpentier and Cerf, 1993; Costerton, 1995; Costerton et al., 1999; Davey y O’Toole, 2000; Kraigsley et al., 2002). Bacteria that are included in a biofilm are in sessile form, exhibiting an altered phenotype from their single or freely suspended (planktonic form) counterparts with respect to growth rate and gene transcription (Donlan, 2002).
Biofilm formation constitutes an efficient adaptive strategy, because such behavior offers four major advantages: (I) protection from adverse environmental factors, (II) increased availability of nutrients for growth, (III) increased binding of water molecules, reducing the possibility of dehydration, and (IV) proximity to progeny and other bacteria, facilitating higher rates of DNA transfer. All these circumstances can increase the survival of bacteria. As a result, inactivation of these bacteria by conventional methods such as use of antibiotics and disinfectants is often ineffective (Costerton et al., 1999; Donlan, 2002).
Biofilms can be found in every environment inhabited by bacteria: natural, industrial or clinical media. Only the presence of a hydrated environment and a minimum amount of nutrients are required, and they can develop on a wide variety of surfaces (hydrophobic or hydrophilic, biotic or abiotic) (Kraigsley et al., 2002; Terry et al., 2003). The presence of biofilms is commonplace in nature. Nowadays, it is believed that in the right environmental conditions, most bacterial species are able to form biofilms (Donlan, 2002; Lasa et al., 2009).
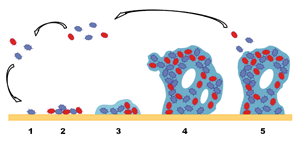
Although the composition of biofilms is variable depending on the system under study, in general the major component of a biofilm is water – up to 97%. Apart from water and the bacterial cells, the biofilm matrix is a complex formed principally by exopolysaccharides (Sutherland, 2001). Moreover, other macromolecules such as proteins, DNA and various products from the lysis of bacteria are present in the biofilm matrix (Branda et al., 2005).Studies of biofilms using confocal scanning laser microscopy have shown that the biofilm matrix architecture is not solid and presents channels that enable water, nutrients and oxygen flow throughout the biofilm. However, the existence of these channels does not prevent different environments inside the biofilm, where the concentration of nutrients, pH, or oxygen varies. This circumstance increases the heterogeneity of the physiological state of the biofilm bacteria and hinders their study (Lasa et al., 2009).Biofilm formation is a dynamic and complex process and different mechanisms are involved in their attachment and growth. Development of a biofilm is not a random process but it is a system that allows prediction (Kumar and Anand, 1998). A common model for the formation of a differentiated and mature bacterial biofilm has been proposed and includes at least five developmental stages. These distinct episodes in the formation of a bacterial biofilm are visualized in Figure 1, and comprise: (i) An initial reversible attachment of planktonic bacteria that approach the solid surface. (ii) Transition from reversible to irreversible attachment by production of extracellular polymers by the bacteria and/or by specific adhesins located on pili and fimbriae, which interact with the surface. (iii) Early development of biofilm architecture. (iv) Development of microcolonies to a mature biofilm. (v) Dispersion of cells from the biofilm into the surrounding environment and return to the planktonic state (Kumar and Anand, 1998).
The presence of biofilms is common in food industry. Biofilms can exist on all types of surfaces in food plants ranging from plastic, glass, metal, wood, to food products (Chmielewski and Frank, 2003).
The attachment of the bacteria to the food product or the product contact surfaces leads to serious hygienic problems and economic losses due to food spoilage. In addition to that, a number of reports have appeared on the persistence of several foodborne pathogens on food contact surfaces. For these reasons, it is considered that the presence of biofilms in the food systems is a serious public health risk.
One of the main problems of the food industry is represented by the survival of foodborne pathogenic or spoilage microorganisms due to an insufficient disinfection of surfaces or instruments that come in contactwith food (Carpentier and Cerf, 1993; Fuster i Valls, 2006).
Biofilms formed on these surfaces are the main cause of contamination of the final product. The consequences of this contamination are rejection of the product, economic losses and even diseases if food-borne pathogens are involved (Piera Serra, 2003). In addition, biofilms formed on the raw meat surfaces and in processing environment (surfaces, tools and instruments...) also offer considerable problems of cross contamination and post-processing contamination. For these reasons food contact surfaces must be sanitized avoiding biofilm formation (Piera Serra, 2003; Fuster i Valls, 2006).
In addition to creating problems associated with public health and product spoilage, biofilms are responsible for mechanical blockages and the impedance of heat transfer processes and increase the corrosion rate of surfaces (Kumar and Anand, 1998; Chmielewski and Frank, 2003).
The formation of biofilms in potable water systems may clog pipes, decreasing velocity and carrying capacity, resulting in increased energy utilization (Chmielewsky and Frank, 2003). Biofilm formation in heat exchangers and cooling towers may also reduce heat transfer and efficiency. The ability of bacteria persist in biofilms formed on metal surfaces in a processing facility could cause corrosion of the surface due to acid-production by the bacteria (Chmielewski and Frank, 2003).
In the dairy industry and other food industries, ultrafiltration and reverse osmosis systems are used during the fractionation of milk and other liquids as well as during the clarification of fruit juices. These membrane filters have very small pores that are continuously in contact with food; the microbial attachment would block the pores and cause silting of the filter. This causes a reduction in the flow with consequent losses of capacity and product (Piera Serra, 2003). Effective cleaning procedures are necessary for the prevention of dangerous and costly damage bacterial biofilms can cause (Silagyi 2007).
The most common foodborne biofilm producers belong to the genera Pseudomonas, Listeria, Enterobacter, Flavobacterium, Alcaligenes, Staphylococcus y Bacillus (Mattila-Sandholm and Wirtanen, 1992; Lee Wong, 1998).
Although many bacteria species are able to form biofilms in the food industry, the most important species in relation to food safety are listed below (González Ribas, 2005).
Listeria monocytogenes
L. monocytogenes is a hardy pathogen with ability to proliferate in cold wet environments that are ideal for biofilm formation. Listeria forms biofilms in pure culture, and can survive and grow in multispecies biofilms(Chmielewsky and Frank, 2003). The most prevalent strain of L. monocytogenes (strain1/2c) found in food processing plants has good adhesion ability and requires only a short contact time for attachment. To initiate attachment, Listeria utilizes flagella, pili, and membrane proteins (Gonzalez Ribas, 2005).
Studies have shown that this pathogen can create a biofilm in slicers and other steel utensils (Keskinen et al., 2008). This fact demonstrates the importance of this biofilm as a factor in cross-contamination. The dairy industry has described the presence of Listeria in milk and dairy products which may be associated with the emergence of clinical outbreaks. It has also been shown that milk protein remains in pipes reduces bacteria, and have a possible inhibitory ability on the biofilm formation in its early stages. However, once established, milk residues in pipes provide a source of nutrients and therefore favour the survival of the biofilm (Lee Wong, 1998).
Salmonella spp.
Among the most virulent foodborne pathogens are Salmonella spp. According to the European Food Safety Authority (EFSA), Salmonella spp. is the most common cause of foodborne outbreaks in the EU in recent years (EFSA, 2009).
Several studies have shown that Salmonella can attach and form biofilms on surfaces found in food-processing plants including plastic, cement, and stainless steel (Joseph et al., 2001; Chmielewsky and Frank, 2003). Salmonella posses a cell-surface appendage (SEF-17 fimbriae) that facilitates adhesion to inanimate surfaces, and provides cells resistance to mechanical forces (Gonzalez Ribas, 2005). Recent studies on the biofilm formation process have revealed that Salmonella and E. coli,as well as many other species of the Enterobacteriaceae family, produce cellulose as a crucial component of the bacterial extracellular matrix and its formation is essential for the survival of the bacteria in the environment (Lasa et al., 2009). Several studies showed significant differences between serovars regarding biofilm formation. The results indicate that the ability to form biofilm is important for the bacteria's persistence in food processing environments. (Vestby et al., 2009).
Escherichia coli
For the formation of biofilms, E. coli uses flagella, pili, and membrane proteins to initiate attachment. After attaching to the surface it loses its flagella and increases the production of extracellular polymeric substance (Gonzalez Ribas, 2005; Houdt and Michiels, 2005).
Studies have found that some strains of E. coli O157: H7 can develop as a result of increased production of exo-polissacharides and curli biofilms (Ryu et al., 2004).
Furthermore, it has been demonstrated that the formation of a biofilm provides greater resistance to E. coli O157: H7 when exposed to solutions of hypochlorite, a frequently used disinfectant in the food industry (Wilks et al., 2005; Ryu and Beuchat, 2005).
Pseudomonas spp.
Pseudomonads are ubiquitous spoilage organisms. They are found in food processing environments including drains and floors, on fruits, vegetables, meat surfaces and in low acid dairy products (Chmielewsky and Frank, 2003; González Ribas, 2005). P. aeruginosa can be considered a model organism for the study of the development of biofilms and its regulation by quorum sensing (Golovlev, 2002). Pseudomonas spp. produce copious amounts of EPS and has been shown to attach and form biofilms on stainless steel surfaces. They coexist within biofilms with Listeria, Salmonella and other pathogens forming multi-species biofilms, more stable and resistant (Chmielewsky and Frank, 2003; González Ribas, 2005).
Campylobacter jejuni
Although Campylobacter does not multiply in food, its minimum infective dose is very low, less than in any other pathogen. In addition, experimental research has suggested that Campylobacter may have greater potential for dissemination during handling of food for the consumer which increases the risk of cross contamination (Joshua et al., 2006; Hanning and Slavik, 2009). One of the mechanisms of survival of Campylobacter spp. in the environment is the formation of biofilms. Campylobacter is capable of producing these biofilms in aquatic media or stainless steel and glass surfaces. The microenvironment created within the biofilm prevents inactivation of C. jejuni by the presence of oxygen. It has been demonstrated that these bacteria are able to survive within the biofilm for a week at 10 ° C, with low nutrient levels and under normal atmospheric conditions. The ability of C. jejuni to develop biofilms faster under aerobic conditions (20% O2) than in microaerophilic conditions (5% O2, 10% CO2), shows the capacity of this micro-organism to adapt the conditions of the biofilm on their behalf, acting as a reservoir of viable cells (Reuter et al., 2010). These studies highlight the role of biofilms for the maintenance of Campylobacter in environments of processed food, increasing the risk of contamination (Murphy et al., 2006).
Bacillus spp.
Bacillus spp. is found throughout dairy processing plants. Bacillus survives heat processing and accumulates in pipelines and joints in the processing environment. If hot fluid continuously flows over a surface for over 16 h, Bacillus and other thermoduric bacteria may form a biofilm (Chmielewsky and Frank, 2003; González Ribas, 2005).
Conclusions
The presence of biofilms in the food industry can be a major problem in terms of technology and public health. The characteristics of this form of bacterial growth involve different behavior regarding cleaning and disinfection processes. Therefore, these formations present a difficulty when a concrete strategy of control has to be chosen.
VISAVET Health Surveillance Centre
Complutense University
References
- Branda S.S., Vik S., Friedman L., Kolter R. (2005). Biofilms: the matrix revisited. Trends in Microbiology, 13, pp: 20 26.
- Carpentier, B., Cerf, O. (1993). Biofilms and their consequences with particular references to hygiene in the food industry. Journal of Applied Bacteriology, 75, pp: 499 511.
- Chmielewsky R.A.N., FranK, J.F. (2003). Biofilm formation and control in food processing facilities. Comprehensive Reviews in Food Science and Food Safety, 2, pp: 22 32.
- Costerton J.W., Stewart P.S., Greenberg E.P. (1999). Bacterial Bioflms: a common cause of persistent infections. Science, 284, pp: 1318 1322.
- Costerton J.W. (1995). Overview of microbial biofilms. Journal of Industrial Microbiology, 15, pp: 137 140.
- Davey M.E., O’Toole G.O. (2000). Microbial biofilms: from ecology to molecular genetics. Microbiology and Molecular Biology Reviews, 64 (4) pp: 847 867.
- Donlan R.M. (2002). Biofilms: microbial life on surfaces. Emerging Infectious Diseases, 8 (9), pp: 881 890.
- EFSA (2009). The Community Summary Report on Trends and Sources of Zoonoses and Zoonotic Agents in the European Union in 2007.
- Fuster i Valls N. (2006). Importancia del control higiénico de las superficies alimentarias mediante técnicas rápidas y tradicionales para evitar y/o minimizar las contaminaciones cruzadas.
- Golovlev E.L. (2002). The Mechanism of Formation of Pseudomonas aeruginosa Biofilm, a Type of Structured Population. Microbiology, 71 (3), pp: 249 254.
- González Ribas F. (2005). Desarrollo y aplicación de sensores para evaluar la contaminación microbiológica de superficies domésticas españolas y de la efectividad de desinfectantes in situ de productos limpiadores comerciales.
- Hanning I., Slavik M. (2009). Campylobacter jejuni as a primary colonizig biofilm former. International Journal of Poultry Science, 8 (1), pp: 1 6.
- Houdt R.V., Michiels C.W. (2005). Role of bacterial cell surface structures in Escherichia coli biofilm formation. Research in Microbiology, 156, pp: 626 633.
- Joseph B., Otta S.K., Karunasagar I, Karunasagar I. (2001). Biofilm formation by Salmonella spp. on food contact surfaces and their sensitivity to sanitizers. International Journal of Food Microbiology, 64 pp: 367 372.
- Joshua G.W.P., Guthrie Irons C., Karlyshev A.V. y Wren B.W. (2006). Biofilm formation in Campylobacter jejuni. Microbiology, 152, pp: 387 396.
- Keskinen L.A., Todd E.C.D., Ryser. E. (2008). Transfer of Surface Dried Listeria monocytogenes from Stainless Steel Knife Blades to Roast Turkey Breast. Journal of Food Protection 71, pp: 176 181.
- Kraigsley A., Ronney P.D., Finkel S.E. (2002). Dynamics of self propagating fronts of motile bacteria.
- Kumar C.G., Anand S.K. (1998). Significance of microbial biofilms in food industry: a review. International Journal of Food Microbiology, 42, pp: 9 27.
- Lasa I., del Pozo J.L., Penadés J.R. (2009). Biofilms Bacterianos e infección.
- Lee Wong A.C. (1998). Biofilms in Food Processing Enviroments. Journal of Dairy Science, 81, pp: 2765 2770.
- Mattila Sandholm T., Wirtanen G. (1992). Biofilm formation in the industry: A review. Food Reviews International, 8 (4), pp: 573 603.
- Murphy C., Carroll C., Jordan K.N. (2006). Environmental survival mechanisms of the foodborne pathogen Campylobacter jejuni. Journal of Applied Microbiology, 100, pp: 623 632.
- Piera Serra, G. (2003). Estudio de biofilms: Formación y consecuencia.
- Reuter M., Mallett A., Pearson B.M., van Vliet A.H. (2010). Biofilm formation in Campylobacter jejuni is increased under aerobic conditions. Applied and Environmental Microbiology.
- Ryu J.H., Beuchat L.R. (2005). Biofilm Formation by Escherichia coli O157:H7 on Stainless Steel: Effect of Exopolysaccharide and Curli Production on Its Resistance to Chlorine. Applied and Environmental Microbiology 7, pp: 247 254.
- Ryu J.H., Kim H., Beuchat L.R. (2004). Attachment and biofilm formation by Escherichia coli O157:H7 on stainless steel as influenced by exopolysaccharide production, nutrient availability, and temperature. Journal of Food Protection 67, pp: 2123 2131.
- Silagyi K.S. (2007). Biofilm formation by Escherichia coli O157:H7.
- Sutherland I. (2001). Biofilm exopolysaccharides: a strong and sticky framework. Microbiology, 147, pp: 3 9.
- Terry A., Curtis E. Pantle R., Penny S.A. (2003). Boundaries for Biofilm Formation: Humidity and Temperature. Applied and Environmental Microbiology, 69, pp: 5006 5010.
- Vestby L.K., Møretrø T., Langsrud S., Heir E., Nesse L.L. (2009). Biofilm forming abilities of Salmonella are correlated with persistence in fish meal and feed factories. BMC Veterinary Research, 5:20.
- Wilks S.A., Michels H., Keevil C.W. (2005). The survival of Escherichia coli O157 on a range of metal surfaces International. Journal of Food Microbiology 105, pp: 445 454.


