Immune recognition and innate response in bovine tuberculosis: summary
Tuberculosis in cattle is a zoonotic disease caused mainly by Mycobacterium bovis, although it is reported outbreaks caused by M. tuberculosis and M. caprae in this specie. In general, it is referring to bovine tuberculosis as the disease caused by the species included in the M. tuberculosis complex (not only by M. bovis). Innate response begins after the infection and before the initiation of adaptive immunity. The importance of this kind of response it is due to its capacity to neutralize the progression of infection.
In cattle, the most probable and important route of infection is by inhalation of the bacteria (even by inhalation of a single bacillus in an aerosol droplet). Ingestion of tubercle bacilli from infected animals or from contaminated pastures, water or fomites is considered secondary to respiratory spread. Congenital infections and vertical transmission are uncommon in regions where intensive eradication programmes operate. Genital transmission can occur if the reproductive organs are infected, but this is extremely rare.
Phagocytic cells play a key role in the innate response to mycobacteria and therefore, in the initiation of the subsequent adaptive T cell immunity. If the route of infection is by inhalation, the alveolar macrophage is the first phagocytic cell involved in the initial uptake of mycobacteria. This cell is included in the group of antigen presenting cells (APCs). Alveolar macrophage tries to destroy mycobacteria through the phagolysosome fusion. If it happens no real infection will take place (animals has the ability to resist the challenge and in most instances this animals will be skin test negative at tuberculin testing). Other animals become infected and progress to exhibit disease. In this case, the alveolar macrophage is destroyed. After the first encounter, dendritic cells and monocytes (other type of APCs) will try to restrain the infection taking part in the phagocytic process. Interestingly, macrophage is the preferred host cell for the intracellular mycobacteria, but it is also a key effector cell for control and destruction of such pathogens.
Experimental studies performed with M. tuberculosis have reported valuable information about immune recognition and phagocytosis of mycobacteria. Both processes are carrying out by different receptor of APCs and are parallel and difficult to summarize (Fig. 1). Toll-like receptors (TLRs) are phylogenetically conserved mediators of innate immunity which are essential for microbial recognition on APCs. In the context of CD14, TLR-2 and TLR-4 binds with lipoarabinomannan and heat-labile cell-associated factor respectively and TLR-9 possibly binds to bacterial DNA. TLRs are expressed not only at the cell surface but also in phagosomes: therefore, immune activation may occur with or without phagocytosis. On the other hand, phagocytosis alone probably does not lead to immune activation without the involvement of TLRs.
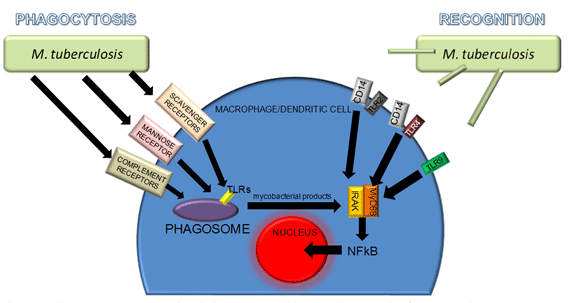
Best characterized receptor for non-opsonin-mediated phagocytosis of mycobacteria is the macrophage mannose receptor, which recognizes terminal mannose residues on mycobacteria. Also, scavenger receptors recognize low density lipoproteins. Phagosomes (product of endocytic pathway initiated by phagocytosis of mycobacteria) can fuse with lysosomes to produce phagolysosomes. The lysosome is a complex vacuolar organelle containing potent hydrolytic enzymes capable of degrading a whole range of macromolecules including microbes. These enzymes function optimally at acidic pH, a condition found within the lysosome (pH 4.5-5.0): this acidic environment is maintained by membrane ATP-dependent proton pumps. Destruction of mycobacteria in the phagolysosomes stimulate activation of IL1-R-associated kinase (IRAK), a serin kinase that activates transcription factors like NF-Κb (nuclear factor kappa B) to signal the production of cytokines. Furthermore, through membrane TLRs, myeloid differentiation protein 88 (MyD88) is activated. MyD88, a common signaling component that links all TLRs to IRAK, is essential for mycobacteria-induced macrophage activation.
Main cytokines in the tuberculosis immune response
Pro-inflammatory cytokines:
These cytokines may inhibit the production or the effects of proinflammatory cytokines in tuberculosis. The most important are:
-
Gamma interferon (IFN-γ): its protective role in tuberculosis is well established, primarily in the context of antigen-specific T-cell immunity. IFN-γ production in vitro after the blood stimulation with mycobacterial antigens is used as a marker of infection. Skin test negative animals do not show purified protein derivative (PPD)-stimulated IFN-γ production in vitro. However, a sonicate of mycobacteria, which contains mycobacterial polyglicans and phospholipids, nonselectively induced IFN-γ production in PPD negative and positive individuals. The cells responsible for this nonspecific production of IFN-γ are Natural Killer (NK) cells, in response to stimulation of different cytokine production by the sonicate. Other cells producers of IFN-γ are lung macrophages and γδ T cells. γδ T cells in cattle (and other ruminants) represent a great proportion of peripheral blood mononuclear cells.
-
Tumoral Necrosis Factor alpha (TNF-α): stimulation of monocytes, macrophages and dendritic cells with mycobacteria or mycobacterial products induces the production of TNF-α. TNF-α plays a key role in granuloma formation, macrophage activation, and has immunoregulatory properties.
-
Interleukin 1-beta (IL-1β): IL-1β is mainly produced by monocytes, macrophages and dendritic cells and it is produced in excess in tuberculosis disease and at the site of disease. IL-1β deficient mice display an increased mycobacterial outgrowth and defective granuloma formation.
-
IL-6: it has both pro- and anti-inflammatory properties. IL-6 can inhibit the production of TNF-α and IL-1β. On the other hand, other studies report an increase in susceptibility infection caused by IL-6 deficiency.
-
IL-12: it is produced mainly by phagocytic cells after the phagocytosis of mycobacteria. IL-12 has a crucial role in the induction of IFN-γ production. The expression of IL-12 receptors is increased at the site of disease.
-
IL-18 and IL-15: in addition to IL-12, both cytokines are important in the IFN-γ axis. IL-18 is synergistic with IL-12 and IL-15 with other cytokines as IL-2, stimulating T-cell and NK-cell proliferation and activation.
Anti-inflammatory cytokines:
These cytokines may inhibit the production or the effects of proinflammatory cytokines in tuberculosis. The most important are:
-
IL-10: it is produced by macrophages after phagocytosis of mycobacteria and after binding of LAM. T lymphocytes are also capable of producing IL-10. Expression of IL-10 antagonizes proinflammatory cytokine response by downregulation of production of IFN-γ, TNF-α and IL-12.
-
Transforming growth factor beta (TGF-β): it is produced in excess by monocytes and dendritic cells during tuberculosis and is expressed at the site of disease. TGF-β supresses cell-mediated immunity: in T cells, it inhibits proliferation and IFN-γ production. In macrophages it antagonizes antigen presentation, proinflammatory cytokine production and cellular activation.
-
IL-4: IL-4 expression causes suppression of IFN-γ production and macrophage activation. In experimental infections, IL-4 expression is related to progressive disease, reactivation of latent and infection and intensified tissue damage.
Chemokines in the immune response against mycobacterias
Chemokines are basic for cell attraction to the site of infection. About 40 chemokines have been identified by now. Some of them have been investigated in tuberculosis:
• IL-8: it is produced by macrophages after phagocytosis of mycobacteria It attracts neutrophils, T cells and monocytes to the site of disease.
• Chemoattractant protein 1 (MCP-1): it is produced by and acts on monocytes and macrophages. Deficiencies of this protein cause inhibition of granuloma formation.
• RANTES (Regulated on Activation, Normal T Expressed and Secreted): It is also known as CCL5. It is produced by a wide range of cell and it binds to multiple chemokine receptors. Expression of RANTES is associated with development of pulmonary granulomas induced by M. bovis.
Other mechanisms involved in eliminating mycobacteria in innate immunity
Certain metabolites and cells help macrophages to kill mycobacteria. Vitamin D, 1,25-dihydroxyvitamin D can supress growth of M. tuberculosis so its deficiency may be a risk factor for the disease.
Another mechanism of defense within phagolysosomes is the production of reactive oxygen intermediates (ROI) and reactive nitrogen intermediates (RNI). Although some mycobacteria are resistant to ROI as superoxyde and hydrogen peroxyde, experimental animals with specific lack in production of these proteins suffer from increase outgrowth of mycobacteria. In regard to RNI, an inhibition of iNOS (nitric oxide synthase) facilitate bacterial outgrowth. In experimental animals, RNI plays a protective role in both acute and chronic persistent infection.
Nramp1 gen codes for natural-resistance-associated macrophage protein (Nramp). It is an integral membrane protein with a role in ionic transport (mainly Fe2+). The gen is involved in macrophage activation and mycobacterial destruction in the phagosomes.
Apoptosis of phagocytic cells may prevent dissemination of infection. The destruction by apoptosis of infected cells reduces the viability of mycobacteria (necrosis do not do it). TNF-α leads apoptosis in response to infection by mycobacteria.
Neutrophils, cytotoxic T lymphocytes (Tc) and NK cells contribute to mycobacterial destruction. NK cells can produce IFN-γ and can be cytolytic towards mycobacteria-infected target cells. Granules of Tc cells and NK cells contain granulysin, a molecule that directly alters the mycobacterial membrane.
VISAVET Health Surveillance Centre
Complutense University
References
Cvetnic, Z., V. Katalinic-Jankovic, B. Sostaric, S. Spicic, M. Obrovac, S. Marjanovic, M. Benic, B. K. Kirin, and I. Vickovic. 2007. Mycobacterium caprae in cattle and humans in Croatia. Int.J.Tuberc.Lung Dis. 11:652-658.
Flynn, J. L. and J. Chan. 2001. Immunology of tuberculosis. Annu.Rev.Immunol. 19:93-129.
Kennedy, H. E., M. D. Welsh, J. P. Cassidy, D. G. Bryson, F. Forster, J. McNair, B. Gangadharan, C. J. Howard, and J. M. Pollock. 2003. The role of WC1(+) gamma delta T-cells in the delayed-type hypersensitivity (DTH) skin-test reaction of Mycobacterium bovis-infected cattle. Vet.Immunol.Immunopathol. 93:169-176.
Pollock, J. M. and S. D. Neill. 2002. Mycobacterium bovis infection and tuberculosis in cattle. Vet.J 163:115-127.
Van Crevel, R., T. H. Ottenhoff, and J. W. van der Meer. 2002. Innate immunity to Mycobacterium tuberculosis. Clin.Microbiol.Rev. 15:294-309.


