Q fever importance in Europe nowadays
Q fever is one of the most widespread neglected zoonoses worldwide, in spite of the fact that it can be considered as emerging or reemerging in many countries [1]. The disease has been described in almost every country where its presence has been investigated [2]with the sole described exception of New Zealand [3,4]. However, many aspects of the Q fever and its causative agent, the obligate intracellular bacterium Coxiella burnetii, remain unclear although in the last years it has received a growing attention that could explain its “emergent” nature (due to the use of improved diagnostic techniques and enhanced vigilance in both Public Health and Animal Health sides).
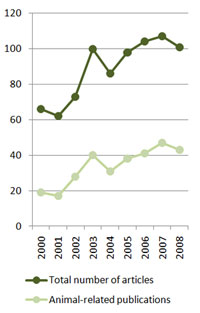
In fact, the number of scientific papers dealing with Q fever has risen from 66 in 2000 to 101 last year; the amount of articles investigating Coxiella burnetii’s importance in animals have doubled in the same period, from 19 to 43 (Figure 2), demonstrating the increasing interest that this pathogen has recently acquired.
In humans, Coxiella burnetii can produce from asymptomatic infections (approximately 60% of cases) to clinical acute or chronic presentations. In acute cases, symptoms include a flu-like syndrome characterized by fever, fatigue and headache, but also atypical pneumonia or hepatitis among others [1,2]. The clinical presentation seems to be influenced by the geographical localization, although the reason for this variation has not been defined yet [5]. In about 5% of human cases the disease may evolve to a chronic form, being endocarditis the most common and severe (often fatal) manifestation of chronic Q fever. Treatment of acute Q fever is often not administered due to absence of diagnosis, although it should be treated when recognized [6]; because of adverse effects of current treatments (mainly doxycycline) children and pregnant women are difficult to treat [2,7]. In chronic cases with endocarditis, life-long treatment with antibiotic combinations and surgery might be needed [6,2].
National Institutes of Health. United States Department of Health and Human Services.
Q fever in animals has been traditionally described as a negligible disease from the clinical and productive point of view, although it can play a significant role in reproductive disorders in livestock [8]. Coxiella burnetii has a broad host range, including mammals, birds and arthropods, and therefore many animal species can act as reservoirs of the pathogen and as sources of infection for human populations, including wildlife. Livestock is considered the major source of infection for humans. Infected animals excrete variable amounts of bacteria on their feces, urine, and milk and especially during abortion or normal delivery, when massive numbers of bacteria are shed to the environment in parturient fluids and infected placentas. Due to the high resistance of C. burnetii to adverse environmental conditions it can survive for weeks, therefore remaining infectious for other hosts. In this way, transmission of the bacteria often occurs after inhalation of aerosols generated from contaminated material. C. burnetii has a very low infectious dose and viable bacteria can be spread by the wind, infecting patients without any evident contact with animals and thus complicating the identification of the source of infection in certain human outbreaks. Although it is not considered primarily as a food-borne zoonosis, other ways of transmission include consumption of unpasteurized milk or milk products. Arthropod-borne transmission has been also described, though it is not considered significant in the epidemiology of the disease [2]. Its natural resistance and low infectious dose make C. burnetii a candidate for being used in bioterrorist attacks, and for this reason it has been included in the category B of Bioterrorism agents list of the Center for Disease Control and Prevention (CDC). Control of the disease in livestock is difficult due to the lack of effective treatments or vaccines. Antibiotics can be used to diminish the incidence of abortions, but they don’t avoid bacterial excretion [9]. Available inactivated phase I vaccines have proven useful in different domestic ruminant species decreasing bacterial shedding under certain conditions [10,11], but vaccines don’t prevent infection.
On the last twenty years several outbreaks of Q fever have been described worldwide, including Europe. Recently, a Q fever outbreak in the Netherlands, starting in 2007 and still ongoing, has affected more than 1500 people so far [12,13]. The most likely source of the outbreak was the presence of infected goat farms localized in the area where it took place, in which management conditions could favor C. burnetii dissemination; dry and windy weather would have helped to spread the bacteria. The magnitude of this episode made necessary to implement important control measures in the Netherlands, as mandatory vaccination campaigns for small ruminants and changes in management procedures (prohibition of spreading of manure in infected farms, checks on animal transport, etc.), and certain concerns on the usefulness of the current strategy have been risen in mass media [14]. The European Centre for Disease Prevention and Control (ECDC) stated that “Vigilance against Q fever may need to be reinforced in the EU” [15].
Several diagnostic strategies can be used for Q fever surveillance, as different techniques for C. burnetii detection are currently available. Clinical diagnosis in domestic ruminants is often difficult, as infection is most of the times asymptomatic, leading to an underestimation of C. burnetii prevalence. Direct detection of the pathogen can be carried out by direct bacterial isolation or detection of its DNA. Culture of C. burnetii is not widely used due to the difficulties handling such a dangerous microorganism, and the need of level 3 biosafety facilities. The other option, amplification of bacterial DNA, is a safe, quick and sensitive method to detect the pathogen, although can only detect infected animals while they are shedding the bacteria. As an alternative to direct detection, indirect diagnosis of Q fever based on detection of specific antibodies in infected hosts (developed usually 2-3 weeks post-infection) is a convenient strategy for screening large number of samples, although interpretation at an individual-level is often difficult. Among the most frequently applied techniques, indirect immunofluorescence assay (IFA) and ELISA offer the highest sensitivity and specificity, and especially the last one can be rapidly automated to analyze large batches of serum samples. On the other hand, a proportion of infected animals that can be shedding the bacteria without showing any clinical sign can be seronegative at these tests and therefore remain undetected.
In summary, there is a lack of knowledge regarding the status of Q fever worldwide, as the disease is not subjected to control programs in livestock and because its incidence in the population is difficult to assess due to the high proportion of asymptomatic infections in human. However, due to its low infectious dose and potential pathogenicity (even causing fatal chronic consequences in a significant number of cases), the puzzle of Q fever and its hosts should be tackled in order to precisely estimate the risk for the general public and prevent future problems. The currently ongoing outbreak in the Netherlands demonstrates the capacity of Coxiella burnetii to cause an important impact in animal and public health when certain changes in animal productive systems are introduced.
VISAVET Health Surveillance Centre
Complutense University
References
1. Arricau-Bouvery, N, Rodolakis,A. Is Q fever an emerging or re-emerging zoonosis? Vet.Res. 2005; 36: 327-349.
2. Maurin, M, Raoult,D. Q fever. Clin.Microbiol.Rev. 1999; 12: 518-553.
3. Greenslade, E et al. Has Coxiella burnetii (Q fever) been introduced into New Zealand? Emerg.Infect.Dis. 2003; 9: 138-140.
4. Hilbink, F et al. Q fever is absent from New Zealand. Int.J.Epidemiol. 1993; 22: 945-949.
5. Cutler, SJ, Bouzid,M, Cutler,RR. Q fever. J.Infect. 2007; 54: 313-318.
6. Hartzell, JD et al. Q fever: epidemiology, diagnosis, and treatment. Mayo Clin.Proc. 2008; 83: 574-579.
7. Tissot-Dupont, H, Raoult,D. Q fever. Infect.Dis.Clin.North Am. 2008; 22: 505-14, ix.
8. Cetinkaya, B et al. Seroprevalence of coxiellosis in cattle, sheep and people in the east of Turkey. Vet.Rec. 29-1-2000; 146: 131-136.
9. Astobiza, I et al. Kinetics of Coxiella burnetii excretion in a commercial dairy sheep flock after treatment with oxytetracycline. Vet.J. 6-3-2009;
10. Guatteo, R et al. Prevention of Coxiella burnetii shedding in infected dairy herds using a phase I C. burnetii inactivated vaccine. Vaccine 12-8-2008; 26: 4320-4328.
11. Arricau-Bouvery, N et al. Effect of vaccination with phase I and phase II Coxiella burnetii vaccines in pregnant goats. Vaccine 15-8-2005; 23: 4392-4402.
12. Delsing, CE, Kullberg,BJ. Q fever in the Netherlands: a concise overview and implications of the largest ongoing outbreak. Neth.J.Med. 2008; 66: 365-367.
13. Schimmer, B et al. Sustained intensive transmission of Q fever in the south of the Netherlands, 2009. Euro.Surveill 2009; 14:
14. van der Lugt, H. Q-fever is killing more people than Mexican flu. NRC Handelsblad 1-9-2009;
15. European Centre for Disease Prevention and Control (ECDC). Q fever: An under-recognised disease that can cause outbreaks. Executive Science Update (Quarterly newsletter for Policymakers) 5-12-2008