Tuberculosis in the new Animal Health Law
Implications of the European Union reference laboratory for bovine tuberculosis in the new mandatory text.

The new Animal Health Law (Regulation (EU) 2016/429) on communicable diseases of animals, which was approved five years ago, will become applicable on the 21st of April 2021. This text, which modifies and repeals certain legislations in the field of animal health, simplifies the priorities in the prevention and eradication of diseases such as bovine tuberculosis.
The regulation also clarifies the responsibilities of all people working directly with animals, such as farmers or veterinarians, and allows a greater use of new technologies in activities related to animal health, such as surveillance of pathogens and electronic identification or registration of animals.
Regarding the legislation for bovine tuberculosis, the Commission has established a series of delegated and implementing regulations to complement and establish clear rules for the application of the 2016/429 regulation (summarized in the following figure).
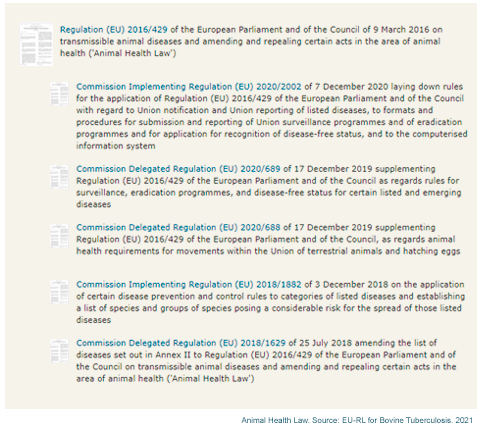
The European Commission's objective is to provide a single and complete law to support the livestock and aquaculture sectors of the European Union. This law will give assistance for a greater competitiveness and a safe European market for animals and their products, which will lead to the development and employment in these important sectors.
A law with a "One Health" approach
The new law adopts a "One Health" approach to improve the early detection and control of diseases, some potentially transmissible to humans, through the establishment of surveillance programs, reducing the occurrence of animal epidemics and offering more flexibility to adjust the rules to local circumstances and emerging issues such as climate and social change. It also improves the legal basis for monitoring antimicrobial resistant animal pathogens.
In relation to tuberculosis, it is referred as infection by bacteria belonging to the Mycobacterium tuberculosis complex, which includes the species M. tuberculosis (more frequently associated with humans) and M. bovis and M. caprae (more frequently associated with animal tuberculosis) (Delegated Regulation (EU) 2018/1629).
The role of the European Union reference laboratory for bovine tuberculosis
The law indicates specific responsibilities for European Union reference laboratories such as the EU-RL for bovine tuberculosis, and establishes the standards that directly affect the control and eradication of the disease.
In relation to the reference laboratory, it must guarantee that the surveillance, control and notification of diseases, eradication programs, the definition of disease-free and the movements of animals and products within the Union, and exports to third countries or territories are based on state-of-the-art, robust and reliable analysis, testing and laboratory diagnostics.
The law states that the EU-RL for Bovine Tuberculosis must provide on its website the operating procedures (SOPs: Standard Operating Procedures) related to the surveillance and control of tuberculosis. Likewise, although the reference laboratory has specific laboratory guidelines on its website, the law indicates the obligation to follow the diagnostic cascade according to article 6 of Delegated Regulation (EU) 2020/689 in case a specific protocol is not available in the EU-RL website.
The Implementing Regulation (EU) 2018/1882, on the application of certain disease prevention and control rules to categories of listed diseases and establishing a list of species and groups of species posing a considerable risk for the spread, establishes the bacteria belonging to the complex Mycobacterium tuberculosis as category E in terrestrial mammals, requiring surveillance within the Union, moreover a category D in artiodactyls requiring certification for intra-community trade and also a category B in the case of bovine, bison and buffalo, requiring eradication.
Finally, it is important to highlight the aspects related to the zoosanitary requirements in the movements of animals within the Union, which are included in Delegated Regulation (EU) 2000/688 were specific guidelines for cattle, goats, camelids and deer are established. Moreover, the Delegated Regulation (EU) 2020/689 includes the surveillance standards, the eradication programs and the tuberculosis-free status.
1VISAVET Health Surveillance Centre
Complutense University Madrid (Spain)
2Animal Health Dpt., Veterinary Medicine Faculty
Complutense University Madrid (Spain)
crlbtb@visavet.ucm.es
More information
- Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health ('Animal Health Law')
- Standard Operating Procedures (SOP) related to the surveillance, control and eradication of animal tuberculosis. VISAVET EU-RL Bovine tuberculosis
- Laboratory methods guidelines for animal tuberculosis diagnosis. VISAVET EU-RL for Bovine tuberculosis
- Bovine Tuberculosis legislation. VISAVET EU-RL for Bovine tuberculosis







